Job Code - 48 (17-08-2020)
(10 times)
Job Code - 47 (29-07-2020)
watch this
Job Code - 46 (28-07-2020)
5 times (Write neatly)
Job Code - 45 (27-07-2020)
5 times (Write neatly)
Job Code - 44 (26-07-2020)
5 times (Write neatly)
Job Code - 43 (25-07-2020)
5 times (Write neatly)
Job Code - 42 (12-07-2020)
5 times (Write neatly)
Job Code - 41 (11-07-2020)
5 times (Write neatly)
Job Code - 40 (09-07-2020)
5 times (Write neatly)
Job Code - 39 (08-07-2020)
5 times (Write neatly)
Job Code - 38 (06-07-2020)
5 times (Write neatly)
Job Code - 37 (05-07-2020)
5 times (Write neatly)
5 times (Write neatly)
Read 3 times Write 5 times
Job Code - 35 (02-07-2020)
5 times (Write neatly)
Read 3 times Write 5 times
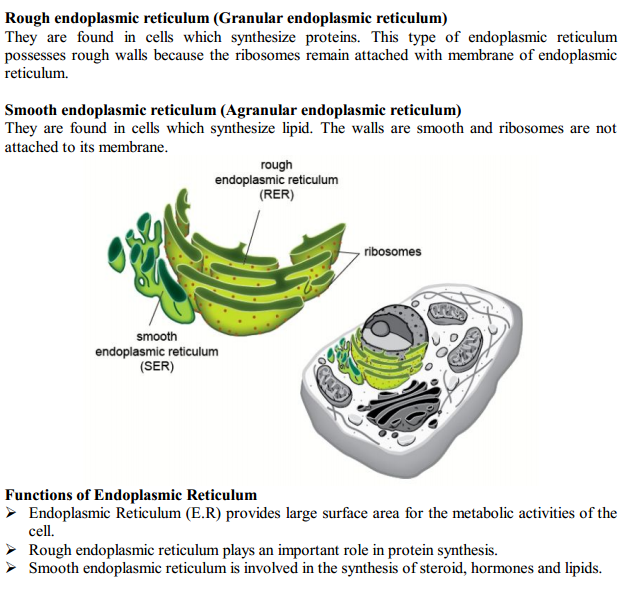
CELL
Cell is called the fundamental unit of life.
A cell is capable of independent existence and can carry out all the functions which are necessary for a living being.
A cell carries out nutrition, respiration, excretion, transportation and reproduction; the way an individual organism does.
Unicellular organisms are capable of independent existence which shows a cell’s capability to exist independently. Due to this, a cell is called the fundamental and structural unit of life.
All living beings are composed of the basic unit of life, i.e. cell.
CELL THEORY (Schleiden, Schwann and Virchow):
• All living organisms are composed of one or more cells.
• The cell is the basic unit of structure, function, and organization in all organisms.
• All cells come from preexisting, living cells.
Job Code - 34 (01-07-2020)
5 times (Write neatly)
Read 3 times Write 5 times
Job Code - 33 (30-06-2020)
5 times (Write neatly)
Read 3 times Write 5 times
Job Code - 32 (29-06-2020)
5 times (Write neatly)
Read 3 times Write 5 times
Job Code - 31 (27-06-2020)
5 times (Write neatly)
Read 3 times Write 5 times
Job Code - 31 (26-06-2020)
5 times (Write neatly)
Read 3 times Write 5 times
Job Code - 30 (25-06-2020)
5 times (Write neatly)
Read 3 times Write 5 times
Job Code - 29 (24-06-2020)
5 times (Write neatly)
Read 3 times Write 5 times
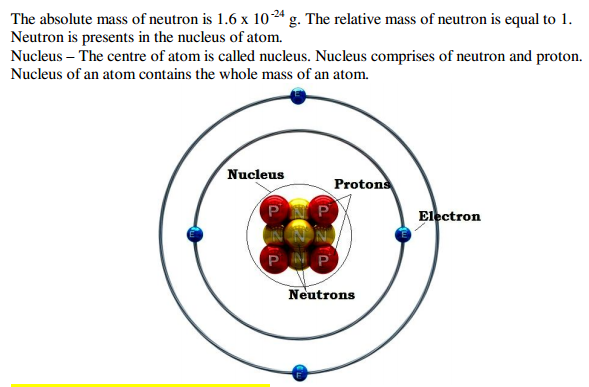
CHAPTER – 4
STRUCTURE OF THE ATOM
Job Code - 28 (22-06-2020)
5 times (Write neatly)
Read 3 times Write 5 times
Job Code - 27 (21-06-2020)
5 times (Write neatly)
Read 3 times Write 5 times
Job Code - 26 (20-06-2020)
3 times (Write neatly)
Read 3 times Write 3 times
Job Code - 25 (19-06-2020)
5 times (Write neatly)
Job Code - 24 (18-06-2020)
10 times (Write neatly)
SUBLIMATION
There are many substances which are converted into gas from solid when heated, and converted from gas to solid when cooled without converting into liquid. Such substances are known as sublime. For example – ammonium chloride, naphthalene balls, camphor,
etc. Therefore, mixture of one sublime and other substance can be separated using the method of sublimation.
The mixture of ammonium chloride and common salt can be separated out using the process of sublimation. For this, the
mixture is heated in a China dish. The China dish is covered by an inverted funnel. Cotton is used for plugging the opening of the funnel. After heating, ammonium chloride is converted into
vapour and gets deposited over the inner surface of funnel; due to cooling. This leaves the common salt in China dish. Ammonium chloride can be taken out by scratching from the inner wall of
funnel.
Job Code - 23 (17-06-2020)
10 times (Write neatly)
SEPARATION OF COMPONENTS OF MIXTURE
CENTRIFUGATION
In the method of centrifugation, the centripetal and centrifugal forces are used to separate lighter and heavier components of mixture of two immiscible liquids. This process is used to
separate very small solids particles from a liquid mixture.
Example – Milk is the mixture of fat, water, and other constituents. Using the method of centrifugation, most of the fat can be separated from milk. In milk, fat is suspended throughout
the milk which is separated out using the method of centrifugation.
When milk is churned rapidly, water which is heavier than fat, migrates away from the centre of centrifuge while fat is forced towards the bottom, which is drained out.
DECANTATION
Decantation is used to separate the components from a mixture of two immiscible liquids, such as mixture of oil and water. In a mixture of two immiscible liquids, lighter one and heavier one
form separate layer. The lighter one can be decanted after settling of mixture, carefully in other container.
In the process of decantation some of the heavier liquid also poured out with lighter one.
Therefore, components from a mixture of two immiscible liquids; can be separated more easily and accurately using a separating funnel.
A separating funnel is usually made of glass with a stop cork with drain pipe at bottom. The heavier liquid which is settled at bottom is drained out from the mixture of two immiscible
liquids by opening of stop cork from a separating funnel.
Job Code - 22 (16-06-2020)
10 times (Write neatly)
SUSPENSION
A suspension is a heterogeneous mixture in which the solute particles do not dissolve but remain suspended throughout the bulk of the medium. Particles of a suspension are visible to
the naked eye.
COLLOIDAL SOLUTIONS
A colloidal solution, occasionally identified as a colloidal suspension, is a mixture in which a substances regularly suspended in a fluid. A colloid is a minutely small material that is regularly spread out all through another substance.
Job Code - 21 (15-06-2020)
10 times (Write neatly)
SATURATED AND UNSATURATED SOLUTIONS
Saturated Solution: When a solution cannot dissolve more solute at a given temperature, the point is called saturation point of the solution and solution is called saturated solution. This means, no more solute can be dissolved in a saturated solution at a given temperature.
Unsaturated Solution: Solution in which more solution can dissolved at a given temperature, is called unsaturated solution.
Solubility: Solubility is the amount of solute in a saturated solution at a given temperature. In other words, maximum capacity to dissolve a solute in a solution at a given temperature is
called solubility.
Different solvents can dissolve different amount of solute. This means different solvents have different solubility Solubility increases with increase in temperature.
Concentration: Concentration is the amount of solute present in a given amount of solvent or solution.
Job Code - 20 (13-06-2020)
10 times (Write neatly)
TYPES OF SOLUTION
Solid - solid solution – Solution of two or more solids are generally known as solid-solid solution. For example – alloys. Alloy is a homogeneous mixture of two or more metals and non
metals or two metals or two non-metals. The components of an alloy cannot be separated by physical methods, their boundaries are not distinct and they can have variable compositions, thus alloy is considered as solution.
Solid – Liquid solution – Solution of solid and liquid is called solid-liquid solution. For
example - solution of salt and water.
Liquid – liquid solution – Solution of two miscible liquids are called liquid-liquid solution,
such as solution of water and acetic acid. The solution of acetic acid in water is known as
vinegar.
Gas - liquid solution – Solution of gas into liquid is called gas-liquid solution. For example –
Soft drink. In soft drink, carbon dioxide is usually dissolved in liquid, because of which a hiss
sound comes while opening the cap of the bottle.
Gas-gas solution – Solution of two or more gas is called gas-gas solution.For example – air,
which is the solution of many gases, such as hydrogen, oxygen, carbon dioxide, etc.
Job Code - 19 (12-06-2020)
10 times (Write neatly)
SOLUTION
Mixture of two or more substances with one phase only,i.e. having no distinct boundary of constituent particles are called solution. For example, solution of sugar and water, solution of
salt and water, lemonade, soft drinks, etc. Solution is a homogeneous mixture of two or more substances.
In a solution, components are mixed in such a way that they appear as only one phase. Seeing by naked eye, constituent particles of a solution cannot be identified because particles are mixed evenly throughout.
In a solution, there are two types of components – oneis called solute and other is called solvent.
Solute – Substance which is present in smaller quantity in a mixture is called solute.
Solvent – Substance in a mixture which is present in larger quantity in a mixture is called solvent.
Example: In the solution of salt and water, salt is present in small quantity while water is present in larger quantity. Here salt is solute and water is solvent.
Job Code - 18 (9-06-2020)
15 times (Write neatly)
HETEROGENEOUS MIXTURE
Mixtures which do not have uniform composition throughout are called Heterogeneous Mixture. For example – mixture of soil and sand, mixture of sulphur and iron fillings, mixture
of oil and water etc. The boundaries of constituent particles of a homogeneous mixture can be identified easily; as a homogeneous mixture has two or m ore distinct phases.
General Properties of Heterogeneous Mixture:
Most of the mixtures are heterogeneous except solutions and alloys.
The constituent particles are present uniformly in a heterogeneous mixture.
The components of a heterogeneous mixture can be identified easily.
Generally, two or more phases are present in a heterogeneous mixture.
Particles of a heterogeneous mixture are sized between one nanometer and one
micrometer or more.
Heterogeneous mixtures show Tyndall effect.
Job Code - 18 (9-06-2020)
15 times (Write neatly)
HOMOGENEOUS MIXTURE
Mixtures which have uniform composition throughout are called Homogeneous Mixture. For example – mixture of salt and water, mixture of sugar and water, air, lemonade, soda water, etc.
Mixture of salt in water is an example of homogeneous mixture. In this mixture, the boundary
of salt and water cannot be differentiated. When a rayof light is passed through the mixture of salt and water, the path of light is not seen.
General Properties of Homogeneous Mixture:
All solutions are the examples of homogeneous mixture.
The particles of a homogeneous mixture are less the one nanometer.
A homogenous mixture does not show Tyndall effect.
The boundaries of particles cannot be differentiated.
The constituent particles of homogenous mixture cannot be separated using centrifugation or decantation.
Alloys are the examples of solution.
Job Code - 17 (8-06-2020)
20 times (Write neatly)
Job Code - 16 (7-06-2020)
20 times (Write neatly)
Job Code - 15 (6-06-2020)
20 times
SUBLIMATION:
The process in which a solid changes into vapor without changing into liquid and from vapor changes into solid without changing into liquid is known as sublimation.
Generally solid first changes into liquid and then changesinto gas because of rise in temperature. But there are many substances, which change into gas without changing into liquid and changes into solid from gas without changing into liquid. Such substances, which go under sublimation, are known as sublime.
For example – camphor, naphthalene balls, ammonium chloride, iodine, dry ice, etc.
The solid obtained after cooling of the gas of sublime is called Sublimate. The process of cooling of vapor of sublime to get sublimate is also known as ‘sublimation’ although it is also known as deposition.
EVAPORATION
The change of liquid into vapor without reaching at its boiling point is called Evaporation.
Evaporation takes place only at the surface of liquid while vaporization takes place on the whole mass of liquid.
Job Code - 14 (5-06-2020)
10 times
Job Code - 13 (4-06-2020)
Dear students from today we will start few chemistry topics.
(10 times)
Job Code - 12 (3-06-2020)
(15 times)
(Each 15 times)
Job Code - 10 (1-06-2020)
Job Code - 9 (30-05-2020)
Physics : Motion Chapter
you need to write 20 times
Job Code - 8 (29-05-2020)
Dear students, we have composed an essential Mathematics work book for you. Today you need to come and collect it and start working on it.
Before coming please give me a call on 9986133697
Nitrogen cycle (10 times)
Job Code - 7 (28-05-2020)
Dear students, MGR Academy is a hard work based institution, completing all the given assignments is very , very mandatory. So, take it seriously.
7 times
Job Code - 6 (27-05-2020)
Dear students, I am giving you biology chapter by chapter work. Once you learn all these it will be very easy for you when we start classes. So, keep doing the assignments even if you don't understand.
Oxygen Cycle (10 times)
Nerve cell (5 times)
5 times
Job Code - 7 (24-05-2020)
Dear students,Today's work (10 times)
Job Code - 6 (23-05-2020)
Dear students,Today's work (5 times)

















































No comments:
Post a Comment